By Philip Clelland
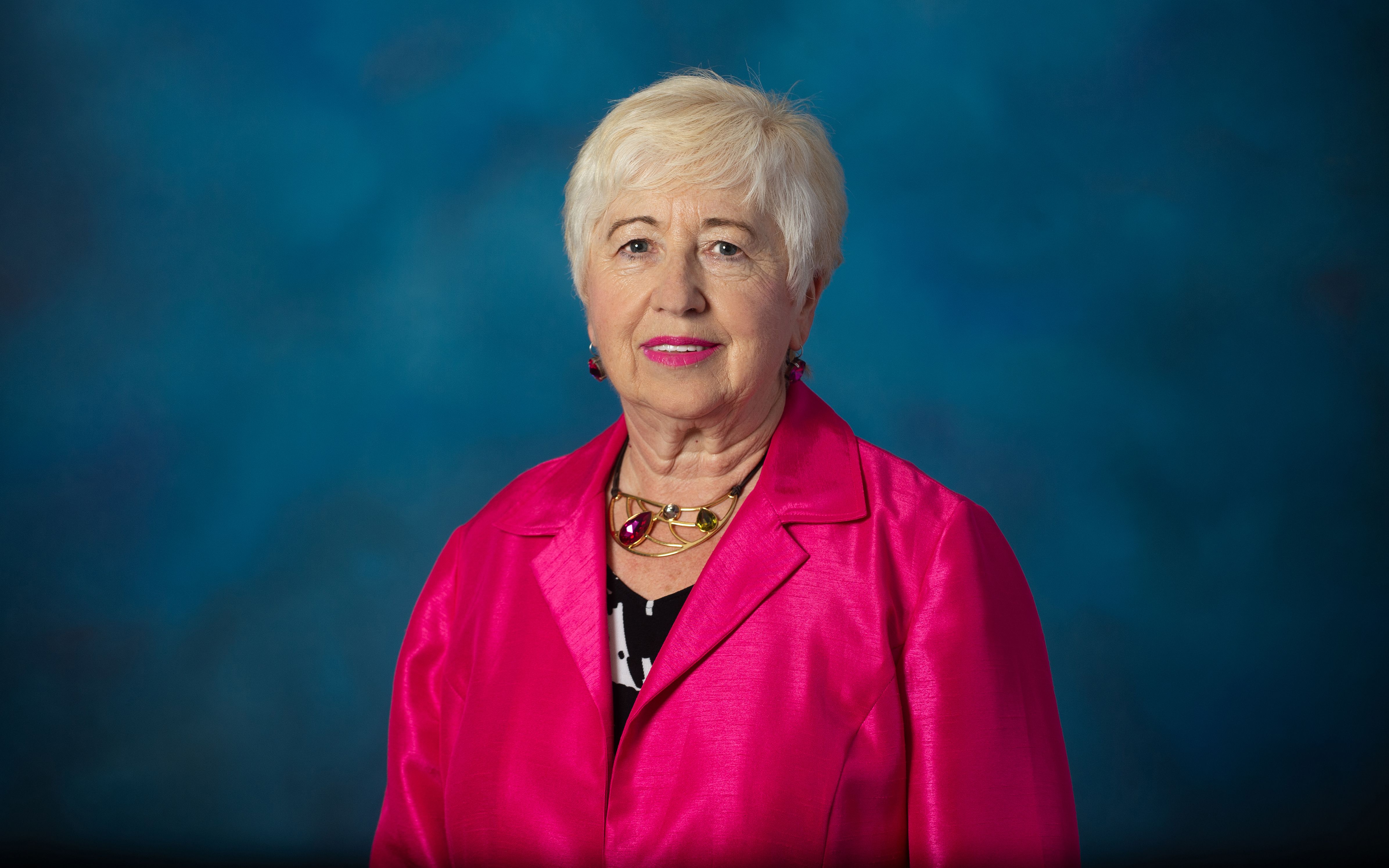 Renowned transplant diagnostics expert Adriana Zeevi, M.D., and her team at the University of Pittsburgh Medical Center leverage NIH funding for research that explores new methodologies to increase patients' opportunities to receive transplants safely.
Renowned transplant diagnostics expert Adriana Zeevi, M.D., and her team at the University of Pittsburgh Medical Center leverage NIH funding for research that explores new methodologies to increase patients' opportunities to receive transplants safely.
"There is tremendous collaboration among multiple institutions because no single center has enough samples to answer the significant global questions in transplantation," Dr. Zeevi says. "NIH recognizes the value—the gold mine—in harnessing multiple centers to rigorously address these questions."
Her groundbreaking studies have analyzed how preformed antibodies affect transplant outcomes, providing clinicians with critical insights into patient management.
NIH funding has supported these complex multi-center collaborations, enabling comprehensive, rigorously controlled studies that private funding typically cannot replicate, she says.
Unlike private sources, NIH funding provides long-term stability, rigorous peer-review processes ensuring scientific validity, and an expansive scope that fosters collaboration across numerous institutions—essential components for addressing significant clinical questions effectively.
"We’re talking about highly sensitized patients who would die waiting for a transplant," Dr. Zeevi says. "Through NIH-funded research, we’ve explored new methodologies and interventions, significantly increasing the opportunities for these patients to safely receive transplants."
 Tina Liedtky, president of Thermo Fisher's transplant diagnostics business
Tina Liedtky, president of Thermo Fisher's transplant diagnostics business
The power of these innovations becomes clear when told through patient stories like that of Amelia Garcia.
Born with severe heart complications, Amelia required a transplant soon after birth, thrusting her parents, Esmeralda and Frankie Garcia, into a daunting journey.
 Baby Amelia Garcia, a heart transplant recipient, is thriving as a result of NIH-funded research.
Baby Amelia Garcia, a heart transplant recipient, is thriving as a result of NIH-funded research.
Today, Amelia is thriving. Her mother joyfully recounts seeing her baby begin to crawl, sit up and develop, reflecting on the transformative power of transplantation. "There's a beautiful side of transplant," Esmeralda says. "Your baby will thrive and grow and learn."
Dr. Zeevi’s research has directly influenced clinical practices that benefit patients like Amelia, drastically reducing invasive procedures and improving the quality-of-life post-transplant. "Advanced diagnostic tools have empowered clinicians to more precisely tailor treatments, improving patient comfort and outcomes dramatically," she says.
 The Garcia Family: Frankie, Esmeralda and baby Amelia.
The Garcia Family: Frankie, Esmeralda and baby Amelia.
Yet, the progress that has saved and enhanced countless lives remains precarious. Both Dr. Zeevi and Liedtky voice concern over uncertainties surrounding federal funding for research agencies like NIH, recognizing how crucial continuous support is for future advancements.
"Clinical trials demand sustained and reliable support," Dr. Zeevi says. "It would be catastrophic to halt funding midway."
Liedtky shares this concern, emphasizing the longer-term risks to innovation. "NIH funding is vital for foundational research," she says. "If it diminishes, the critical pipeline for innovation stalls, limiting our ability to deliver solutions that transform patient lives."
For families like the Garcias, the real-world implications are clear. "A transplant doesn’t just save one life—it saves entire families," Esmeralda says.
This interconnected ecosystem of academic research, federal support, and industry innovation underscores why sustained NIH funding is not merely budgetary—it's a lifeline. Dr. Zeevi sums it up succinctly: "It's not simply about science. It's about translating research into real-world solutions. It's about saving lives."
| Government Relations contact: | Media contact: |
| ken.monahan@thermofisher.com | media.relations@thermofisher.com |

